Ever wanted to know the science behind your favorite silicone lube?
A lubricant is essential in anal intercourse for both tops and bottoms. Sadly, the anal passage is not able to lubricate itself, and therefore if you start raw-dogging without anything to reduce friction, tearing can occur.
The rectal lining is a bit fragile so if you’ve got some thick, veiny cock hammering into you, it can tear the lining and make you more susceptible to sexually transmitted infections. And if you’re topping, the dick itself can develop tears along the shaft. Again, that can lead to infection, and distress.

A do-it-all lube kit from sexual wellness brand Cake. With so many lube options, it can be easy to become overwhelmed.
So, lubrication is crucial, and most people appreciate that silicone-based lubricants are the perfect lubricant for anal intercourse. But why, you may ask?
What makes silicone slippery?
Before I overload you with info I’ll quickly go through some terminology:
Organosilicon compounds: A molecular compound made of silicon-carbon bonds.
Siloxane: An organosilicon compound made of a silicon atom attached to two oxygen atoms (O-Si-O) as well as two carbon branches (methyl groups to be accurate i.e. CH3). They can make long chains (polymers) or rings (cyclic).
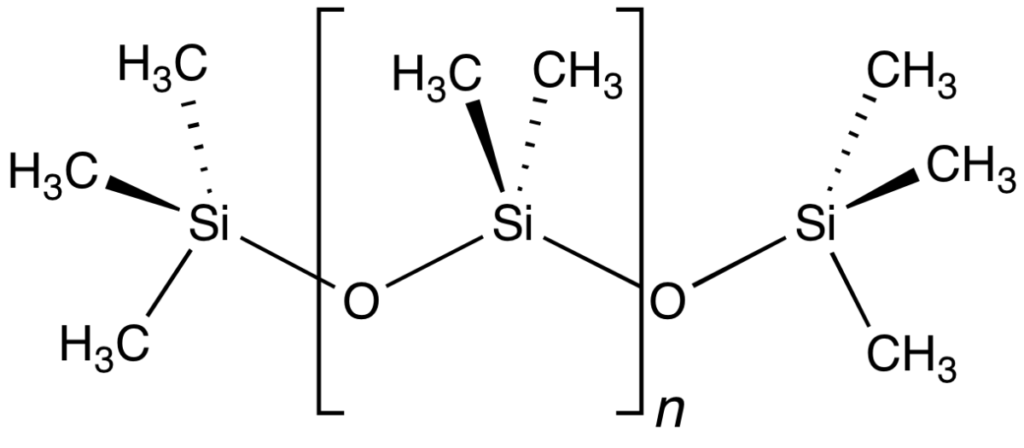
Polydimethylsiloxane (commonly known as dimethicone) is a useful polymer of siloxanes. The brackets between the Si and O indicate it is repeating. The ‘n’ represents the number of times this repeats. (Public Domain)
Polymers: a long molecular chain made of subunits of repeating molecules. These can be natural, such as DNA or synthetic like silicones.
Silicones: Polymers of siloxane subunits.
Tetrahedral geometry: A chemical geometry of one central atom with four bonded atoms. Each of the atoms forms an even angle with its neighbor (109.5°). This gives it low potential energy and keeps them extremely stable.
Silicone is slipping around everywhere
Silicones are a highly multifunctional class of chemicals. They are used industrially in automotive, electronics, coatings, building, medicine, personal care and lubricants (including intimate lubricants).
Some of the cool properties that these compounds have are: they’re water resilient, they slip and glide easily, can protect from chafing, and last a long time on the skin.
How does it do this? Because silicones are made up of tetrahedral geometric structures of silicon and oxygen as its backbone. This makes them very resilient and unreactive. “Unreactive” is exactly what you want when it’s on your ding dong and in your hole.
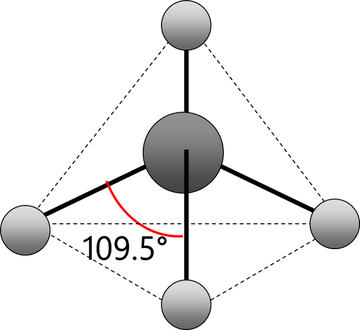
A diagram of tetrahedral chemical geometry. Each atom forms a 109.5° angle with its neighbors. This structure is only applicable if there are no lone pairs on any of the atoms and the four “arms” are the same substituent (Public Domain)
So silicones you regularly find in your lube are: dimethicone, dimethiconol, and cyclopentasiloxane.
Combating Silicone Shade
There’s some silly misinformation out there about silicones primarily around toxicity, degradability and breathability. One of my favorite science communicators Dr. Michelle Wong of Lab Muffin Beauty did a wonderful breakdown of these misconceptions. To summarize:
Toxicity: The current consensus on silicones is that they are considered safe for use in intimate lubricants. This assessment has been made by multiple regulatory bodies: the Cosmetics Ingredient Review (US), EU Scientific Committee on Consumer Safety, Health Canada, UK Environment Agency and the Australian Industrial Chemical Introduction Scheme.
Degradability: Although it is true that silicones are not biodegradable, this term means that living things can’t break it down into its substituent particles. However! They can be broken down into silica (sand), carbon dioxide, and water through binding to clays and sediments mostly in wastewater treatment facilities. Environmental toxicity of these silicones are currently assessed to be of little concern. The only compounds that are restricted are cyclic silicones (i.e. cyclopentasiloxane) and only in the EU. (The EU operates on a precautionary model based on lab studies rather than environmental assessment.)

Decamethylcyclopentasiloxane (commonly known as cylcopentasiloxane) is slightly more volatile than dimethicones due to its smaller size and ring formation. It is also referred to as D5. It is assessed safe at restricted doses in wash-off products in the EU. (Public Domain).
Breathability: One of the most pervasive myths on silicone is that its film-forming properties can reduce breathability or permeability. This is completely counter to how they operate: silicones are actually permeable to oxygen and water vapor. Dr. Wong postulates that this myth comes from how silicones are used in longwear products which gives a false perception that they cannot be removed. But, luckily, skin sheds a layer each day.
RELATED LINK: All The Fun That’s Inside Cum
It all comes down to what’s getting lubed up!
Silicones are your best friend in anal sex, but they are but one type of lube ingredient. There’s oil-based lubes (not your friend if you wear latex condoms, as they can be damaged by the lube) and water-based lubes (not ideal for long play, but some industrial ones that can be made from powder are preferred for fisting).
Silicones are super fun for your home use, I do hope this has illuminated just a little about the cool chemistry that helps you slide your way into a sexy twink or hunky daddy. Buy a lube that you feel most comfortable with, and that is able to accommodate your play. Be safe, have fun, slide in!
Sources: Australian Industrial Chemical Introduction Scheme, Cosmetic Ingredient Review, Environmental Science & Technology, EU Scientific Assessment on Consumer Safety, Health Canada, International Journal of Toxicology, Lab Muffin Beauty Science
Get real time update about this post category directly on your device, subscribe now.



